Why Is Ice Slippery? By Lama 3 Gradient
This is the answer given by llama3-gradient:8b-instruct-1048k-q6_K
Why ice is slippery?
A simple but fundamental question!
Ice is slippery because of the way it melts. As you may know, when water is turned into a solid (frozen) state, it loses volume and becomes less dense than its liquid form. This means that ice cubes float on top of water because they are lighter.
When a solid (like wood or metal) is placed on top of another surface, it can only grip the surface with as much friction as the two surfaces have in common. For example, if you put a rock on a piece of paper, there’s very little to no grip between them and the rock will slide easily because their atoms are not interacting closely enough. Similarly, when you step on ice orother slippery material, your shoes don’t have any real grip with the surface either.
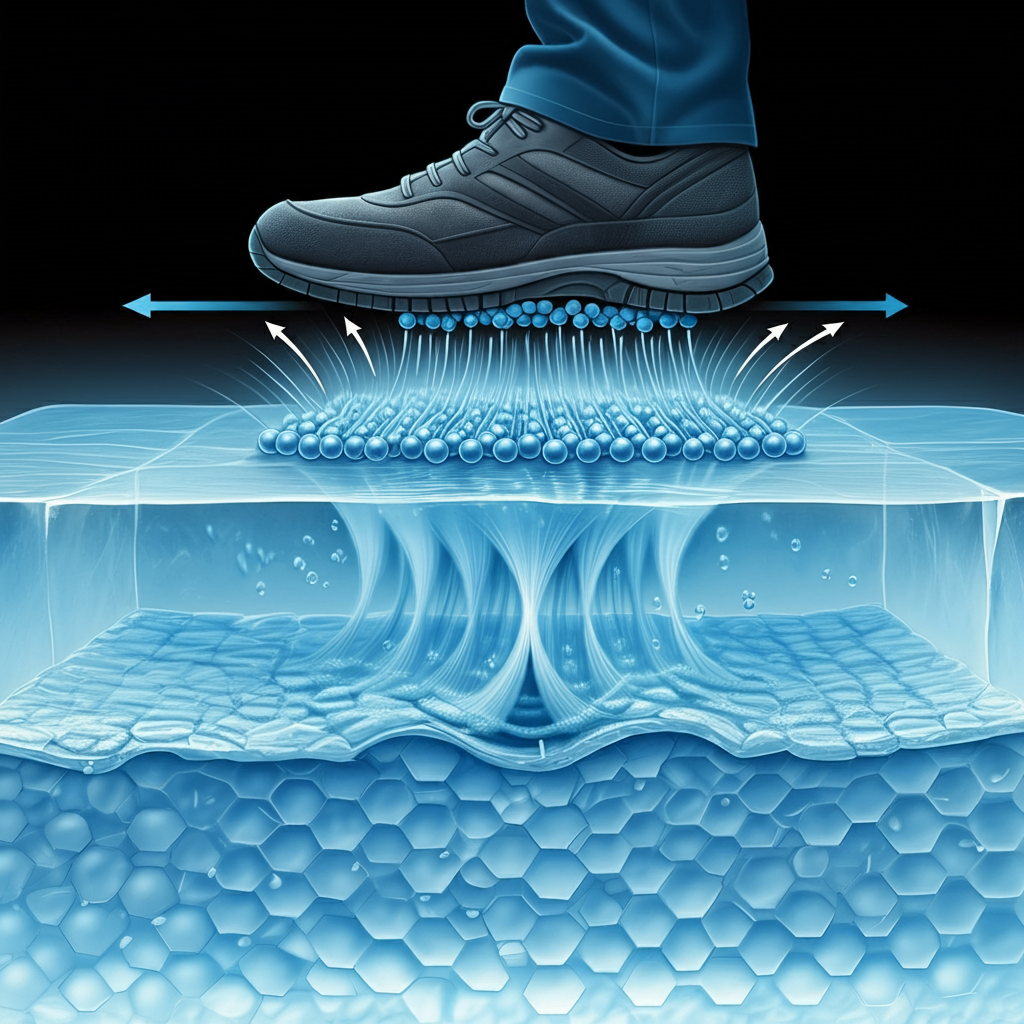
However, water is different in this regard. When it freezes into a solid (ice), the molecules that make up water are arranged in a crystalline structure called hexagonal close-packed array (or a lattice). In this arrangement the molecules of the crystal lattice are very tightly packed so there is minimal space between them. If you push one molecule, several others will move along with it. The key point here is the “very tightly packed” bit.
When pressure is applied to an ice surface by a foot or tires, the tiny gaps in the structure allow water molecules at the very top of the lattice to be pushed and they can’t find any more solid molecules behind them to push against. As this happens, the ice surface that was initially a very flat plane starts to form into a “brush” (or even a series of “brooms” if you like). The pressure causes water molecules to get forced out of their normal lattice position and move upwards by one or two molecules, but they can’t go any further because there aren’t enough more in front of them. As the pressure is released the molecules at the very top will fall back into place as soon as possible.
So now you have a “brush” instead of a flat surface with some sort of grip between the ice and the solid (like your shoes or car tires). The brush will push against any force that comes against it in such a way that the force is spread out over many more molecules than if the force were acting on a very small area, like if you just pushed down hard on a single molecule.
This means that there’s less pressure per unit of ice surface than if your foot or tire had to push against a solid object, which would be pushing against one specific spot.
In other words, it is easier for your weight and the weight of the car to push against many molecules than just the right ones on a flat surface. So, instead of the force being concentrated in one area (as you could expect from all the tiny water molecules) it spreads out so that there’s very little pressure at any point. In physics we call this “lubrication”.
When you try to stop or change direction while your car is moving on ice, the weight of the vehicle is spread over many more molecules than if you were on a solid surface. The weight is distributed so it takes much less force for the friction (or grip) between the tires and the ice to be overcome than if you were on a solid surface.
Also, when the road is covered in snow or water, the melting has only occurred at the very top of the molecules. The layer underneath that is still just like the original “brush”. So even with the weight spread out over many more molecules, there are no solid molecules to push against and you end up slipping a little as your car tires do not have enough grip on the ice. In other words, they don’t get any purchase.
So in short, when an ice surface is melted from the top down (which it will always be when you are driving or walking because friction generates heat), there are many more molecules at the very top than if a solid were to be pushed against by your weight and tires. So the force that pushes against the “brush” is spread out so much that it takes much less pressure to overcome the friction for your foot or tire to slip over the ice surface. It’s not a lot different from pushing an entire bunch of feathers with one finger versus pushing just a single feather.
This is why ice is slippery when you walk on it or try to stop your car on it, but it will take much more pressure to push against the molecules at the top of the brush than if they were all arranged in their usual lattice (as they are when the surface is solid).
So there’s another factor that can make ice even slipperier. As the temperature drops, the molecules at the very top of the “brush” become a bit more rigid and less likely to move when pushed against. This increases the friction by making it harder for you to push against many of them all at once with one force. But this is not much different from pushing a single feather – as soon as the pressure is released, they return to their original “brush” shape. So even though there’s more molecules in your way than if you were on a solid surface, it takes less force for them to get out of your way again.
So when you walk or drive, it is easier to push against all these water molecules and the weight is spread out so much that it doesn’t take much friction (or grip) for your foot or tire to slip over the ice. Also as you slide along, there’s no solid surface to push against under your shoes and tires because even though they are only a few micrometers thick, all of them are already melted from the top down!
So if you ever drive on a road covered in black ice (or driving too fast), slow down or stop slowly. If you walk across an icy surface, try to put most of your weight on one foot. That way you can make use of the friction by pushing against only that small part of the brush rather than the entire brush.
(For all of this to happen there must be some melting of the top layer of ice. This is what I mean by “melting from the top down” – it means that the temperature of the surface (the very top) is lower than the bottom. It’s a bit different if you walk on frozen water, in which case the entire thickness of the ice is solid and not so slippery.)
And remember when there are other forces at work such as the force of friction from a tire against a road or the force your feet make as they push against the brush (like the force to get up after a fall – there’s no grip then either). If you were able to take a single feather and push it, but only with one end, all the feathers at the other end would be pushed because the top ones would not move. But when your foot pushes, the entire “brush” is pushed against – so even though many more molecules are in your way than if you were pushing just a single feather, they’re easy to push through and won’t give much grip.
Also, it’s not as slippery when there’s some ice in the middle because then all those water molecules on top are supported by solid ones. In other words, the brush has two “feathers” (the bottom of the brush) instead of just one so your weight is spread out across more molecules and they’re easier to push against than a single feather.
In summary, the reason ice is slippery is because when you put pressure on it, the force you apply gets spread out over many more molecules than if you were pushing a solid. The top layer is easy to move and as soon as you do, there aren’t any solid molecules at the very top (because of melting) or just below (when there’s ice in the middle), so your weight will easily be overcome by that force.
I hope this makes sense!
(Note: In a similar way, when you are sliding on an ice rink, the blades of the skates can push against many more molecules than if they were pushing one spot. That’s why the pressure is less and it takes much less friction to keep your feet moving in the same direction that you started out with. So there is much less force needed for them to overcome the friction – but at least on an ice rink, there are other forces also working against you like air resistance and rolling resistance. This is why figure skaters have such a hard time doing jumps or spins – they need more force than is normally required from just gliding.)
This is all quite basic. The details of the physics get much more complicated as soon as we talk about different types of ice (like very cold, extremely cold, black ice etc.) but this is why a normal solid surface like wood or metal has good friction and a “brush” such as water on top has none at all. This is one reason why a car tire will slip over the brush of water on an icy road and how you can make your feet grip on the brush when you walk in a cold winter morning – if there’s some snow or water, your feet have something to push against.)
Oh! And I almost forgot. The same thing happens with friction between two surfaces (like car tires and a road). If the two surfaces are very smooth, so that there isn’t much grip for the molecules of one surface to interact with those on the other, it’s hard for them to resist each other. So if you’re going over a smooth surface like ice or water or even just an extremely smooth asphalt (like the kind they use at the Indy 500), your car tires won’t have as much grip and the car will move faster in any gear than it would on a rougher road.
(Note: I know that this is simplified – physics can get very complicated and many other factors come into play. But I tried to keep it simple.)